are “made in EU” nutraceuticals more successful?
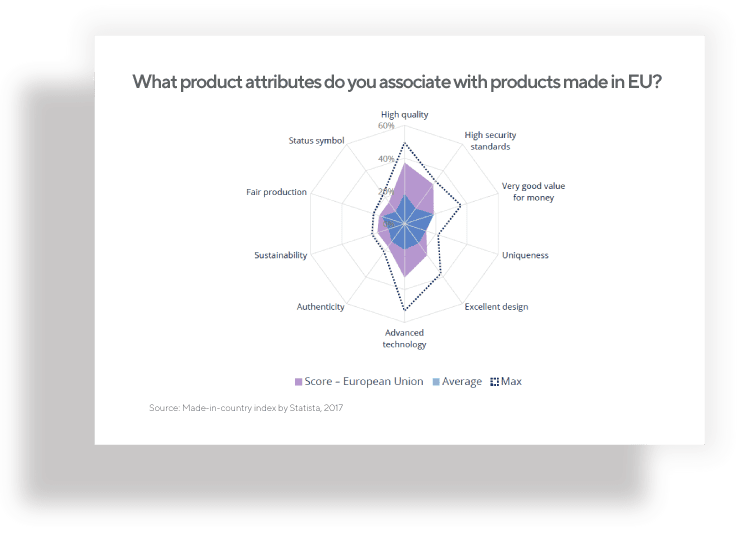
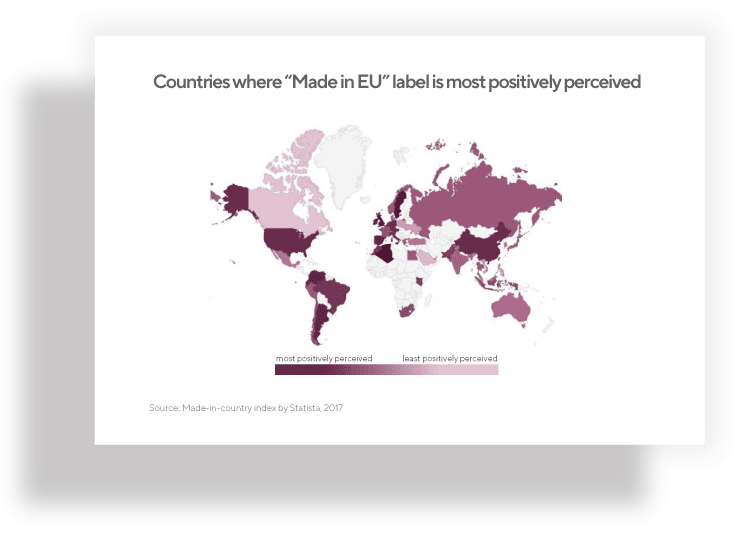
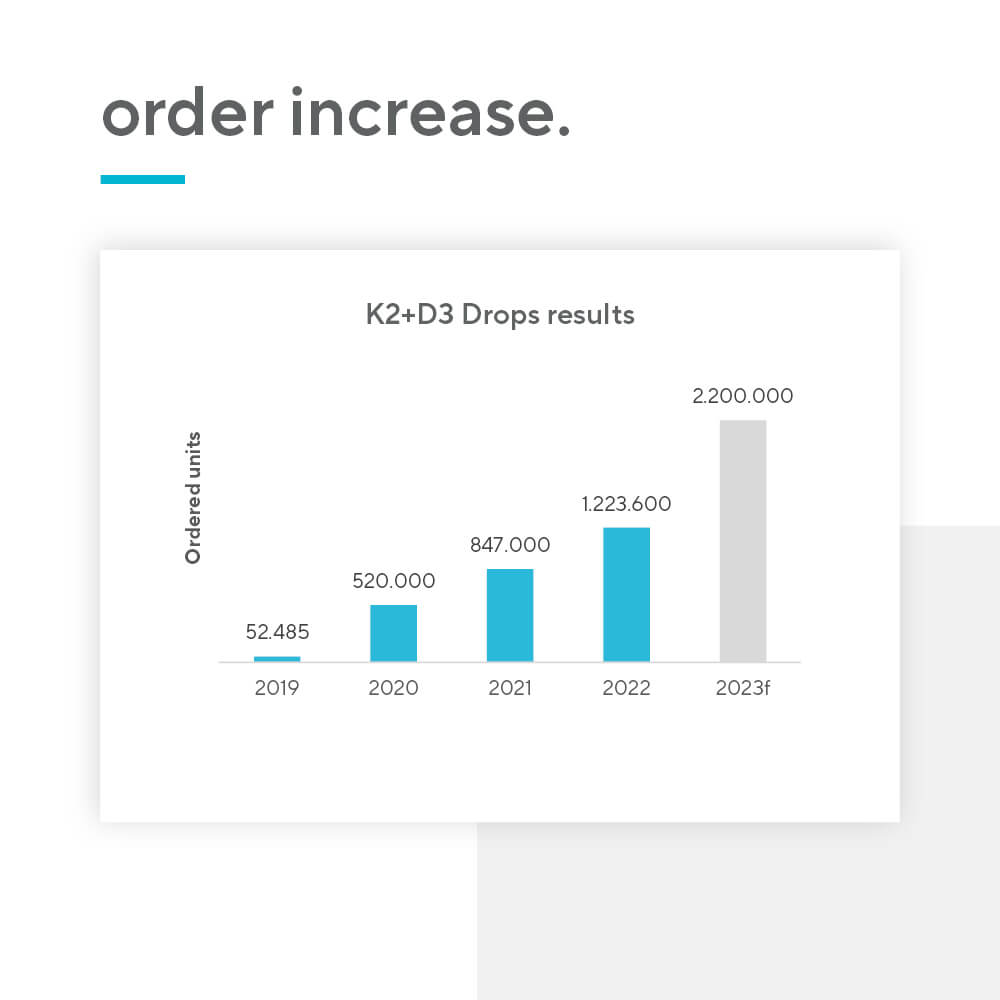
Many factors can influence how your nutraceutical product will be accepted among consumers and in the food supplement industry. The “Made in EU” label aspect is shown to create a more positive product perception, especially among non-European consumers. By choosing a private label supplement produced in the European Union for your next product launch, you may improve your chances for success.